We all start as egg cells in our mother’s ovaries. Scientists have been working on generating in vitro models for the ovaries so that we can study more about them since the female reproductive organs are surprisingly understudied till now.
Scientists at the Wyss Institute for Biologically Inspired Engineering at Harvard University, Harvard Medical School (HMS), and Duke University, in collaboration with Gameto, report that they have created a living, fully human ovarian organoid that supports egg cell maturation, develops follicles, and secretes sex hormones.
The ‘ovaroid’ model allows the study of ovarian biology without even extracting the tissue sample from the patient’s body and this enables to design and development of new treatments for conditions like infertility, ovarian cancer, and many more. The technology has been licensed to Gameto, which is used to develop different forms of treatments for disorders related to the female reproductive system. The meaning of ‘ovaroid’ is described as “Directed differentiation of human iPSCs (Induced Pluripotent Stem Cells) to functional ovarian granulosa-like cells via transcription factor overexpression” in eLife.
More about the model
An in vitro model of human ovarian follicles is very helpful in studying the female reproductive system from many different aspects. For the proper development of the ovary, a combination of germ cells and multiple types of somatic cells are required.
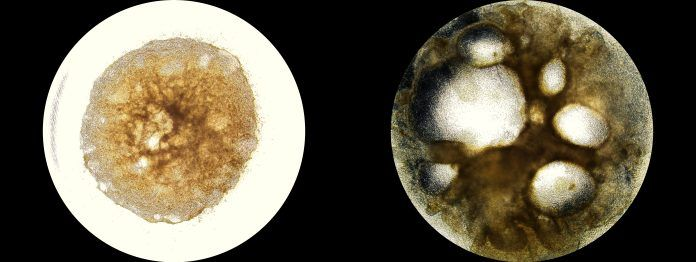
The granulosa cells (a type of cell in ovaries that produces the hormones like estrogen and progesterone) Plays a major role in follicle formation and oogenesis. Whereas efficient protocols exist for generating human primordial germ cell-like cells (hPGCLCs) from human induced Pluripotent stem cells (hiPSCs), a method of generating granulosa cells has been elusive,” write the investigators.
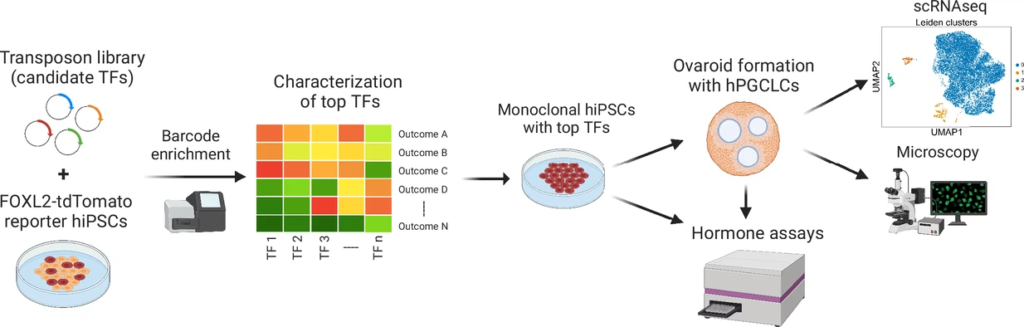
The simultaneous expression of two transcription factors (TF) can direct the difference between hiPSCs to granulosa-like cells. They have illustrated the regulatory effects of some of the granulosa-related TFs and established that overexpression of NR5A1 and either RUNX1 or RUNX2 is enough to generate granulosa-like cells. Our granulosa-like cells have some transcriptome factor which is very much similar to the human fetal ovarian cells and summarize the ovarian phenotypes including follicle formation and steroidogenesis.
When our cells are aggregated with hPGCLCs it will lead to the formation of ovary-like organoids (ovaroids) which support the hPGCLC development from the premigratory to the gonadal stage as measured by induction of DAZL (deleted in azoospermia-like: plays key role in germ line establishment and gametogenesis) expression. This model provides multiple opportunities to study the female
reproductive system in more detail.
The co-first author Merrick Pierson Smela said that “Our new method of fully human ovaroid production is several times faster than existing human/mouse hybrid methods, and replicates many of the critical functions of these organs, marking a significant step forward in our ability to study female reproductive health in the lab. In the future, similar technology could also treat infertility by growing egg cells from people whose own eggs aren’t viable“.
“Creating the granulosa cells on their own was a significant accomplishment, but making an ovaroid out of only granulosa cells wouldn’t tell us anything about their ability to support the maturation of germ cells, which was what we wanted to be able to study in vitro,” said co-first author Christian Kramme. This process had been done previously by using hPGCLCs and mouse somatic cells, but with this new technology they can make it with the complete ‘human model’. The team from Wyss is still developing the human ovaroid model and plans to integrate it with the other ovarian cell types like hormone-producing the cells to copy the complex function of the human ovary. They are improving their culture
system to make their germ cells into the fully developed egg cell. Meanwhile, Gameto is conducting preclinical research on a derived co-culture system for egg maturation in humans with leading national fertility clinics.